068-522-75-40
063-890-85-41
063-799-20-91(viber)
0-800-31-89-87
(calls within Ukraine are free)
044-334-89-87
Mode of operation
Mn. – Fr.: 9:00 - 17:00
-



COVID-19
-



Hepatitis B
-



Hepatitis C
-



Hepatitis A
-


Toxoplasmosis
-


Rubella virus
-


Cytomegalovirus infection
-



Herpes simplex virus
-



Epstein-Barr virus
-


Varicella-Zoster virus
-


Mumps virus
-


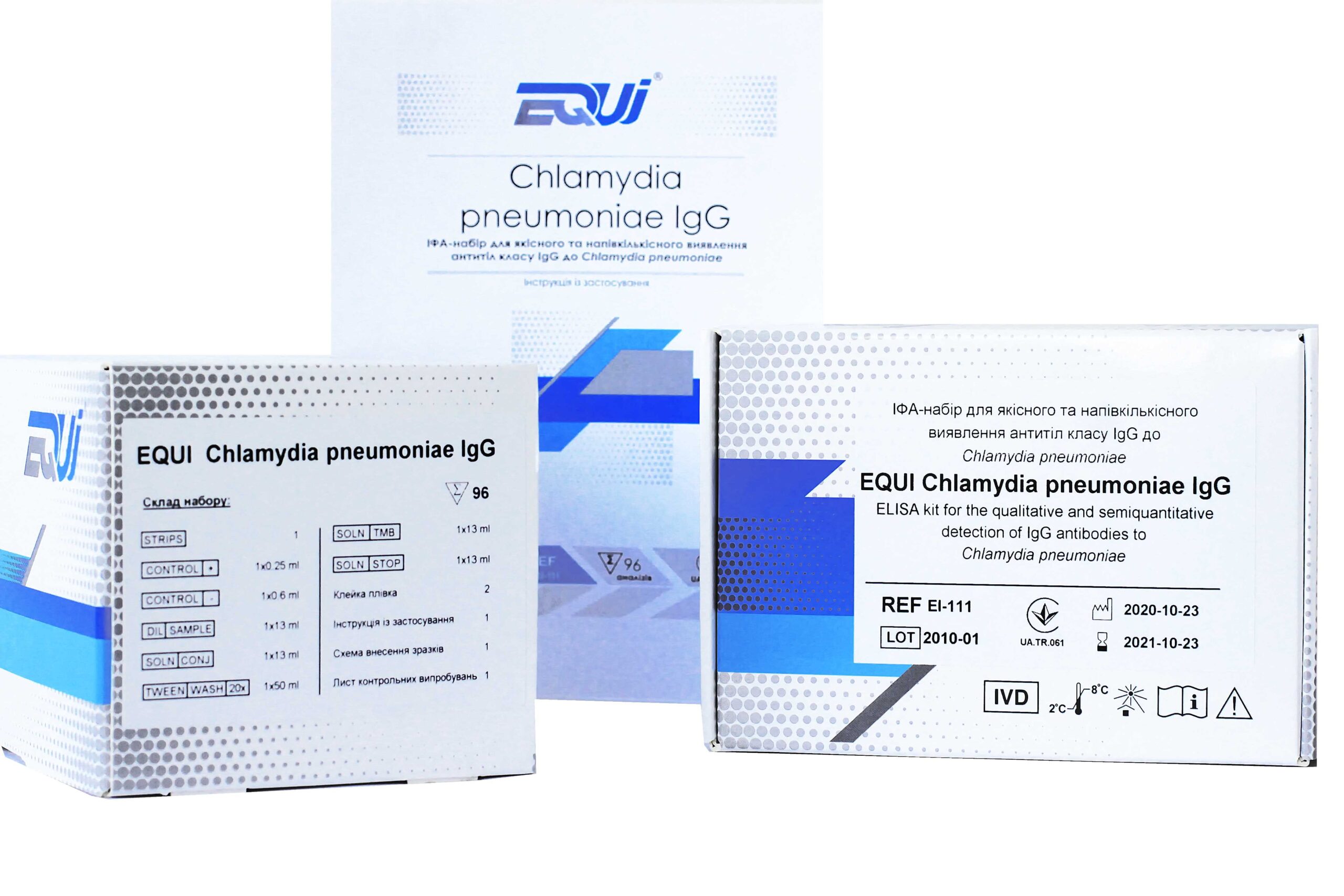
Urogenital chlamydia
-



Measles virus
-



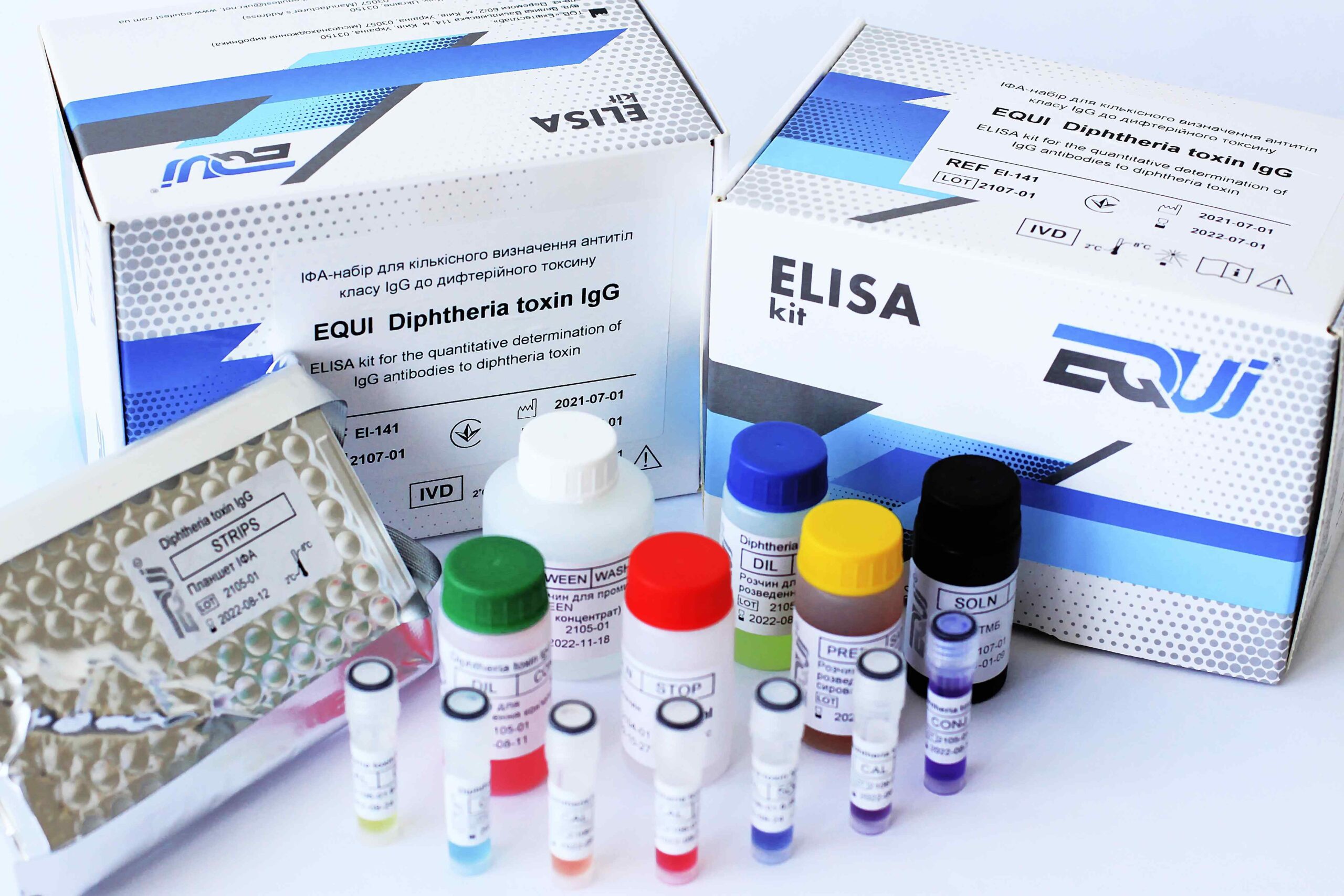
Diphtheria
-



Urogenital mycoplasmosis
-



Ureaplasmosis
-



Trichomoniasis
-



Pathogens of human parasitic diseases
-



Gastrointestinal infections
-



Syphilis
-


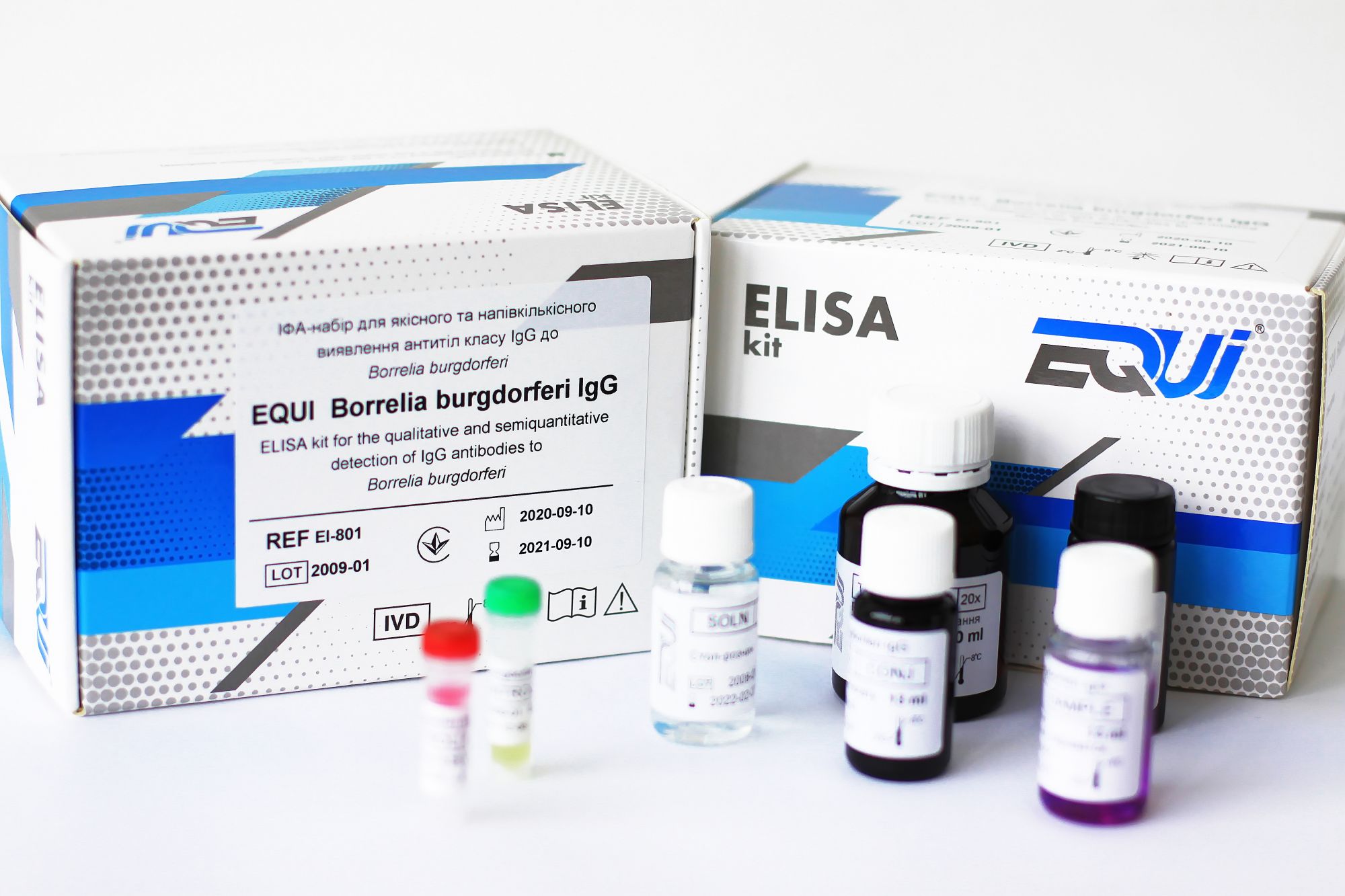
Borreliosis
-



Bordetella pertussis toxin
-



Allergy